 |
|
Exercise 2. Read all chemical formulas used in the texts.
Exercise 3. Make an oral translation of the text.
Text 7
Industries
Mining
Froth flotation is a process for separating minerals from gangue by taking advantage of differences in their hydrophobicity. Hydrophobicity differences between valuable minerals and waste gangue are increased through the use of surfactants and wetting agents. The selective separation of the minerals makes processing complex (that is, mixed) ores economically feasible. The flotation process is used for the separation of a large range of sulfides, carbonates and oxides prior to further refinement.
Waste water treatment
The flotation process is also widely used in industrial waste water treatment plants, where it removes fats, oil, grease and suspended solids from waste water. These units are called Dissolved air flotation (DAF) units. In particular, dissolved air flotation units are used in removing oil from the wastewater effluents of oil refineries, petrochemical and chemical plants, natural gas processing plants and similar industrial facilities.
Principle of operation
Froth flotation commences by comminution (that is, crushing and grinding), which is used to increase the surface area of the ore for subsequent processing and break the rocks into the desired mineral and gangue in a process known as liberation, which then has to be separated from the desired mineral. The ore is ground into a fine powder and mixed with water to form a slurry. The desired mineral is rendered hydrophobic by the addition of a surfactant or collector chemical. The particular chemical depends on which mineral is being refined. As an example, pine oil is used to extract copper. This slurry (more properly called the pulp) of hydrophobic mineral-bearing ore and hydrophilic gangue is then introduced to a water bath which is aerated, creating bubbles. The hydrophobic grains of mineral-bearing ore escape the water by attaching to the air bubbles, which rise to the surface, forming a foam or a scum (more properly called a froth). The froth is removed and the concentrated mineral is further refined.
Science of flotation
To be effective on a given ore slurry, the surfactants are chosen based upon their selective wetting of the types of particles to be separated. A good surfactant candidate will completely wet one of the types of particles, while partially wetting the other type, which allows bubbles to attach to them and lift them into a froth. The wetting activity of a surfactant on a particle can be quantified by measuring the contact angles that the liquid/bubble interface makes with it. For complete wetting the contact angle is zero.
Another consideration, especially important for heavy particles, is to balance the weight of the particle with the surfactant adhesion and buoyant forces of the bubbles that would lift it.
For typical values of metal densities and surface tensions, if the bubbles are larger than the ore particles, and the particles are equal to or less than 1 mm, then particles will rise into the froth layer if:

where  is the radius of the particles,
is the radius of the particles, 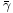 is the average surface tension between the three pairs of phases (particle, flotation solution, air),
is the average surface tension between the three pairs of phases (particle, flotation solution, air),  is the mass density of the particles, and
is the mass density of the particles, and  is the acceleration of gravity (9.81 m/s2).
is the acceleration of gravity (9.81 m/s2).
For particles larger than the bubbles, they too can rise into the froth, each buoyed by a swarm of bubbles, under similar conditions as those expressed in the inequality.
Mechanics of flotation

Simple flotation circuit for mineral concentration. Numbered triangles show direction of stream flow, various flotation reagents are added to a mixture of ore and water (called pulp) in a conditioning tank. The flow rate and tank size are designed to give the minerals enough time to be activated. The conditioner pulp [1] is fed to a bank of rougher cells which remove most of the desired minerals as a concentrate. The rougher pulp [2] passes to a bank of scavenger cells where additional reagents may be added. The scavenger cell froth [3] is usually returned to the rougher cells for additional treatment, but in some cases may be sent to special cleaner cells. The scavenger pulp is usually barren enough to be discarded as tails. More complex flotation circuits have several sets of cleaner and re-cleaner cells, and intermediate re-grinding of pulp or concentrate.
Pyrometallurgy
Pyrometallurgy is a branch of extractive metallurgy. It consists of the thermal treatment of minerals and metallurgical ores and concentrates to bring about physical and chemical transformations in the materials to enable recovery of valuable metals. Pyrometallurgical treatment may produce saleable products such as pure metals, or intermediate compounds or alloys, suitable as feed for further processing.
Examples of elements extracted by pyrometallurgical processes include the oxides of less reactive elements like Fe, Cu, Zn, Chromium, Tin, Manganese.
Pyrometallurgical processes are generally grouped into one or more of the following categories:
- Drying
- Calcining
- Roasting
- Smelting
- Refining
Most pyrometallurgical processes require energy input to sustain the temperature at which the process takes place. The energy is usually provided in the form of fossil fuel combustion, exothermic reaction of the material, or from electrical heat. When enough material is present in the feed to sustain the process temperature solely by exothermic reaction (i.e. without the addition of fuel or electrical heat), the process is said to be "autogenous."
Drying
Drying is thermal removal of liquid moisture (not chemically bound) from a material. Drying is usually accomplished by contacting the moist solids with hot combustion gases generated by burning fossil fuels. In some cases, heat for drying can be provided by hot air or inert gas that has been indirectly heated. The amount of heat required for a given drying operation corresponds to the heat required to vaporize the liquid moisture, the heat required to raise the temperature of the products (dry solids and water vapor) to the final drying temperature, and heat required to offset radiant heat losses.
Usually the drying temperature is set at a nominal value above the boiling point of water, often about 120°C. In special cases, such as in the drying of certain water-soluble salts, higher drying temperatures are required. In salt drying, the feed moisture is saturated with dissolved salts, which alters the boiling point and requires higher drying temperatures.
Drying of moist solids is carried out in several types of industrial dryers, including rotary dryers, fluidized bed dryers, and flash dryers.
Another type of drying, called spray drying, is carried out when the material to be dried is completely dissolved in aqueous solution. The solution is sprayed (usually through a specially designed nozzle) into a heated chamber and as the water is evaporated, solids crystallize. The water vapor is exhausted from the dryer, and dry solids are collected, usually in a conical section of the dryer. Solid material produced from a spray dryer often has special particle size and shape characteristics, which may be controlled by the concentration of dissolved material in the solution, and the design of the atomizing spray nozzle.
Calcining
Calcining is thermal decomposition of a material. Examples include decomposition of hydrates such as ferric hydroxide to ferric oxide and water vapor, or decomposition of calcium carbonate to calcium oxide and carbon dioxide and or of iron carbonate to iron oxide. Calcination processes are carried out in a variety of furnaces, including shaft furnaces, rotary kilns, and fluidized bed reactors.
Roasting
Roasting consists of thermal gas-solid reactions, which can include oxidation, reduction, chlorination, sulfation, and pyrohydrolysis.
The most common example of roasting is the oxidation of metal sulfide ores. The metal sulfide is heated in the presence of air to a temperature that allows the oxygen in the air to react with the sulfide to form sulfur dioxide gas and solid metal oxide. The solid product from roasting is often called "calcine." In sulfide roasting, if the temperature and gas conditions are such that the sulfide feed is completely oxidized, the process is known as "dead roasting." Sometimes, as in the case of pre-treating reverberatory or electric smelting furnace feed, the roasting process is performed with less than the required amount of oxygen to fully oxidize the feed. In this case, the process is called "partial roasting," because the sulfur is only partially removed. Finally, if the temperature and gas conditions are controlled such that the sulfides in the feed react to form metal sulfates instead of metal oxides, the process is known as "sulfation roasting." Sometimes, temperature and gas conditions can be maintained such that a mixed sulfide feed (for instance a feed containing both copper sulfide and iron sulfide) reacts such that one metal forms a sulfate and the other forms an oxide, the process is known as "selective roasting" or "selective sulfation."
Smelting
Smelting involves thermal reactions in which at least one product is a molten phase.
Metal oxides can then be smelted by heating with coke or charcoal (forms of carbon), a reducing agent that liberates the oxygen as carbon dioxide leaving a refined mineral. Concern about the production of carbon dioxide is only a recent worry, following the identification of the enhanced greenhouse effect.
Carbonate ores are also smelted with charcoal, but are sometimes need to be calcined first.
Other materials may need to be added as flux, aiding the melting of the oxide ores and assisting in the formation of a slag, as the flux reacts with impurities, such as silicon compounds.
Smelting usually takes place at a temperature above the melting point of the metal, but processes vary considerably according to the ore involved and other matters.
Refining
Refining is the removal of impurities from materials by a thermal process. This covers a wide range of processes, involving different kinds of furnace or other plant.
The term, 'refining' can also refer to certain electolytic processes. Accordingly, some kinds of pyrometallurgical refining are referred to as 'fire refining'.
Words to know:
1. gangue
2. surfactant
3. wetting agent
4. suspended solid
5. effluent
6. to commence
7. liberation
8. slurry
9. pulp
10. foam
11. contact angle
12. conditioning tank
13. rougher cell
14. scavenger
15. barren
16. circuit
17. drying
18. calcining
19. roasting
20. smelting
21. refining
22. radiant heat losses
23. rotary dryer
24. fluidized bed dryer
25. flash dryer
26. aqueous solution
27. nozzle
28. to evaporate
29. furnace
30. shaft furnaces
31. rotary kiln
32. fluidized bed reactor
33. gas-solid reaction
34. reverberatory
35. flux
Exercise 1. Explain the meaning of the following words and word combinations:
Subsequent, to aerate, surfactant, barren, saleable product, drying, calcining, roasting, smelting, refining, to sustain.